Our research agenda focuses on two primary themes:
1. Role of the α7β1 integrin as a mechanosensor and intrinsic modulator of myofiber growth following exercise and mechanical strain
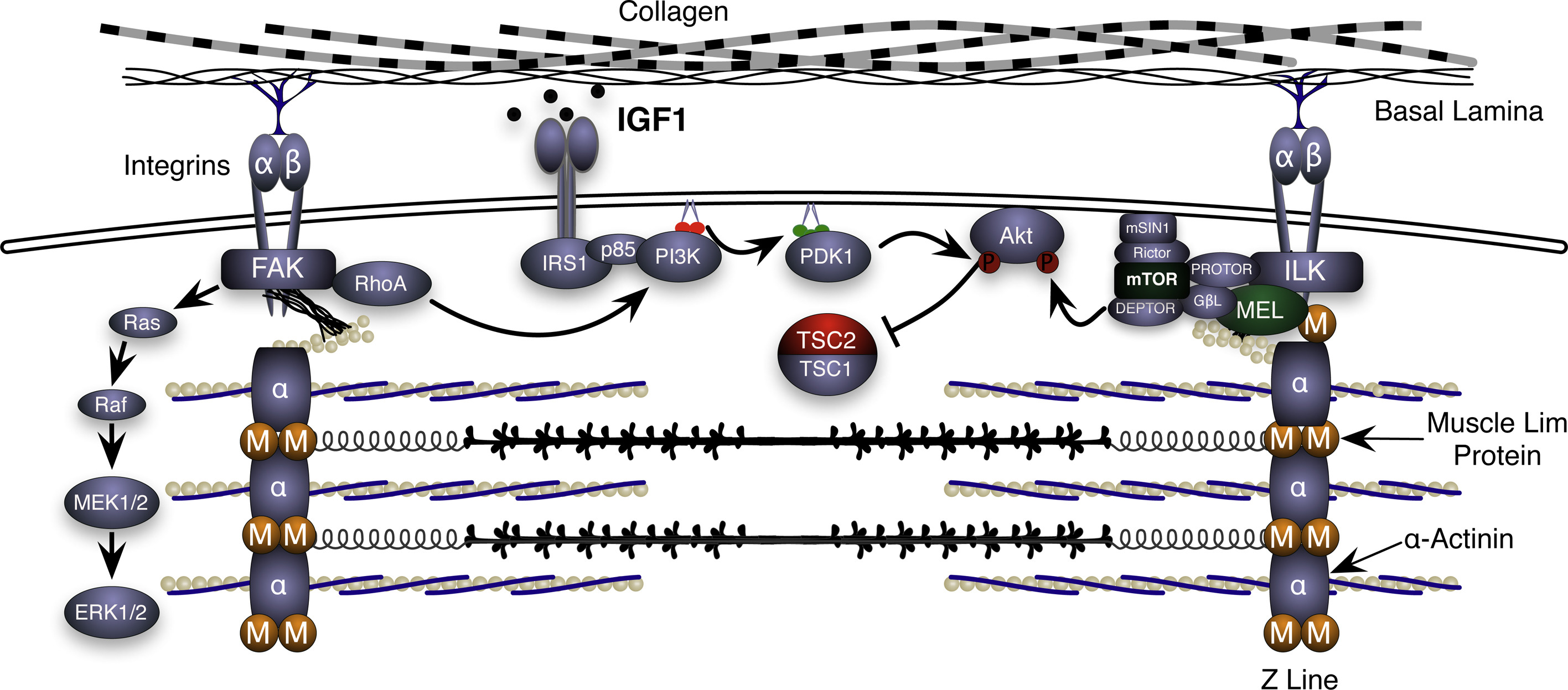
Objective – The α7β1 integrin is a heterodimeric transmembrane receptor that links laminin on the outside of the muscle fiber to the actin cytoskeleton on the inside of the cell. Our laboratory was the first to document that α7 integrin RNA and protein are increased in both rodent and human skeletal muscle following an acute bout of eccentric exercise (Boppart 2006, Boppart 2008, De Lisio 2015). We subsequently used MCK:α7B integrin transgenic and α7 integrin knockout mice to demonstrate that this important integrin subunit can protect skeletal muscle from strain-induced sarcolemmal damage and facilitate muscle growth following both eccentric exercise (Boppart 2006, Boppart 2008, Lueders 2011, Zou 2011) and chronic loading.
Current efforts are focused on understanding the mechanistic basis by which the α7β1 integrin complex can promote strain-induced muscle growth (Mahmassani 2017). In addition, important clinical questions are addressed, such as whether loss of integrin localization and/or function provides the basis for delayed myofiber growth observed with natural aging or following a prolonged period of immobilization.
2. Development of cell-based strategies to effectively recover skeletal muscle mass and strength following disuse
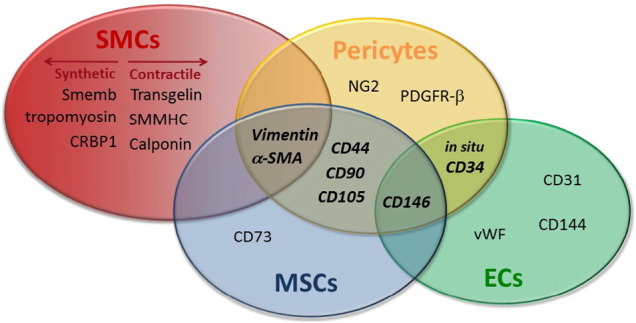
Skeletal muscle supports a reservoir of mononuclear cells that collectively maintain tissue structure and function. Satellite cells serve as the primary myogenic progenitor, yet other resident stem/stromal cells in the perivascular niche may contribute to tissue repair, remodeling and growth. Perivascular stem/stromal cells include both pericytes and mesenchymal stem cells. In 2012, we documented an increase in perivascular stem/stromal cell quantity in skeletal muscle following an acute bout of exercise (Valero 2012). We have subsequently reported sensitivity of these cells to mechanical strain and the ability to for transplanted cells to facilitate repair, myofiber growth, arteriogenesis, and strength in the context of exercise in mice (Huntsman 2013, Zou 2015).
Current efforts are focused on identifying muscle-resident mononuclear cells that are sensitive to both the presence and absence of mechanical strain using single cell RNA sequencing. Our goal is to exploit the mechanosensitive nature of these cells to develop cell- and cell-free (extracellular vesicle-based) strategies to effectively recover muscle mass and strength following disuse, and ultimately prevent long-term disabilities in older adults.